 Stainless Steel & High Tensile Steel Bolts & Quality Parts |
Home About Technical & FAQ How To Buy & Contact Terms & Conditions of Sale Links |
|

8:8 Bolt

10:9 Bolt
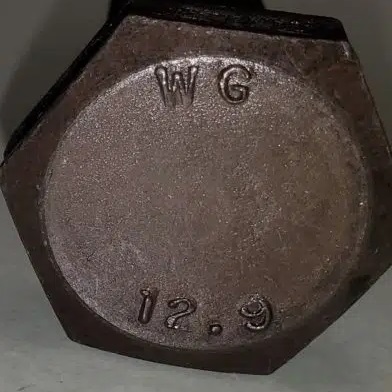
12:9 Bolt

A4-80 Bolt

A2-70 Bolt
Product Catalogues
VW Split Screen Van
VW Bay Window Van
VW Beetle
VW Type 3
VW Thing / Trekker
VW Karmann-Ghia
VW Engines T1+T4
VW Electrical
IGNITION SYSTEMS
TOOLS
Product Catalogues
Air Cooled Porsche
Rover V8 Parts
Ford Kits
BMC Era Car Parts
Land Rover Kits
What Is A2-70 Stainless Steel ?
|
DIN/ISO A2 Stainless Steel Is A Corrosion Resistant Steel; It Is Also Known As ASTM-304. A2 / ASTM-304 Is An 18/8 Stainless Steel : This Designates A Metallurgical Content Of 18% Chromium & 8% Nickel. A Bolt Marked A2-70 Is A 304 Stainless Steel Bolt With A 700 N/mm2 Tensile Strength (See Below Table). A2 / 304 Stainless Steel Is What Is Known As An Austenitic Stainless Steel : It Is (Mostly) Non-Magnetic. A2 / 304 Stainless Steel Is One Of The Most Highly Corrosion-Resistant Materials Available To The Designer And Engineer. |
How Do A2-70's Material Properties Compare To OEM Mild Steel Bolts ?
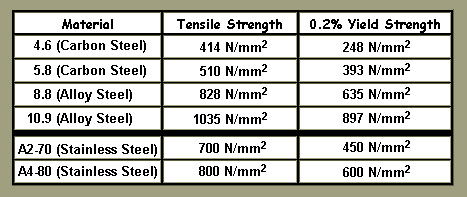 Tensile Strength Denotes The Load At Which The Material Breaks. 0.2% Yield Strength Denotes The Load That Will PERMANENTLY Deform / Stretch The Material By 0.2% Of Its Original Size. As Can Be Seen : A2-70 Stainless Exceeds Ordinary Low Grade Steels For Strength. Though It Is Weaker Than 8:8 Heat Treated Steels, Which Are Quite Commonplace. All Bolt Heads Will Have A Strength Code Or Reference Forged, Stamped Or Engraved On To Them For Identification. Any Bolt WITHOUT Markings Shall Be Considered To Be Of The Most Inferior Grade - Unless Its Composition Is Supported By Paperwork. Sometimes "Specials" Are Made By Turning Hex Bar, For Example. 
Nuts Will Also Have Markings On Them For Identification. Washers Do Not Carry Any Such Markings |
Where Can I Use A2-70 Stainless Steel Bolts ?
|
A2-70 Can Be Safely Used To Hold Car Body Panels And Bumpers; Wing (Fender) Bolts, Hinge Bolts, Handles, Locks, Light Fittings Etc. A2-70 Can Be Safely Used On Non-Mechanically Loaded Engine Components Where A Simple Clamping Action Is Required (Subject To Torque Limitations). A2-70 Can Also Be Used For Securing Access Panels, Cover Plates And Accessory Items To The Vehicle. |
Where Should I NOT Use A2-70 Stainless Steel Bolts ?
|
A2-70 Should Not Be Used In Heavily Loaded Mechanical Or Structural Areas.
Any Nut Or Bolt Stamped With A 10:9 Or 12:9 Should Be Replaced With An Identically Rated (Or Higher) Component AND NOT An A2-70 Component. The Below Table Gives A Rough Guide To The Maximum Torque Wrench Settings To Be Used On The Most Popular Metric Sizes.  |
What Is The Difference Between A2 & A4 Stainless Steel ?
|
A4 Grades Of Stainless Steel Are Usually Reserved For Use In Highly Caustic Environments Where Improved Resistance To Pitting Corrosion Is Required, i.e Marine & Exhausts. The Improved Resistance Comes From The Addition Of A Higher Proportion Of Chromium, Which Helps To Form A Protective Oxide Layer On The Iron - The More Chromium You Have The Longer The Protection It Offers Lasts. However, Whilst Some Properties Are Improved, Others Deteriorate. The Extra Chromium Makes The Metal More Succeptable To Fatigue And Cracking : OK If You Are Rolling Serenly Around On The High Seas : Not So OK If You Are Driving An Old Car Like A VW.! This Extra Protection Is Not Necessary For Automotive Use As The Component Life Of A2-70 Grades Is Generally Massive (Lifetime Guarantees Are Often Given On Items Made From These Materials). Professional Engineers Agree That A2 Stainless Is The Optimum Selection For Automotive Use. |
How Much Of An Issue Is Galvanic Corrosion ?
|
Galvanic Corrosion Takes Place When Two Dissimilar Metals Are Electrically Coupled In The Prescence Of An Electrolyt : The Electrolyt Assisting
In The Transfer Of The Charged Atoms / Molecules Necessary For The Corrosion To Take Place. The Below Picture Shows A New Zinc Annode Fitted Next To A Spent Annode On A Canal Boat. The Rate At Which Galvanic Corrosion Can Proceed Is Goverened By Two Key Factors.
Stainless Steel Is Less Reactive Than Ordinary Mild Steel Or Cast Irons ; Therefore The Iron/Steel Will Corrode Galvanically (If The Above Conditions
Are Met) Protecting The Stainless Steel. |


